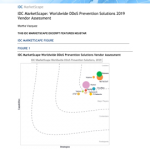
See a live demonstration of the capabilities of Appian’s solution for the study design and execution lifecycle.
COVID-19 has greatly disrupted the clinical study lifecycle, with many studies having to be placed on hold or halted and others needing to be accelerated in a race for COVID-19 treatments. It is crucial now more than ever to efficiently and effectively manage the multitude of changes that are necessary to meet current study requirements. Appian’s flexible solution built on our low-code automation platform gives you the ability to customize operations to meet your changing needs as well as accommodate evolving regulatory requirements.
Watch this webinar for an interactive discussion and live demonstration of the capabilities of Appian’s solution for the study design and execution lifecycle, including:
- Process automation across the initiation, kickoff, and coordination of the study design and execution workflow
- Enhanced visibility and traceability through unified interfaces and heightened reporting
- Seamless connectivity and integrations across systems and processes throughout the study lifecycle














Add Comment