Learn how Appian gives you the power to improve the business impact of your clinical studies.
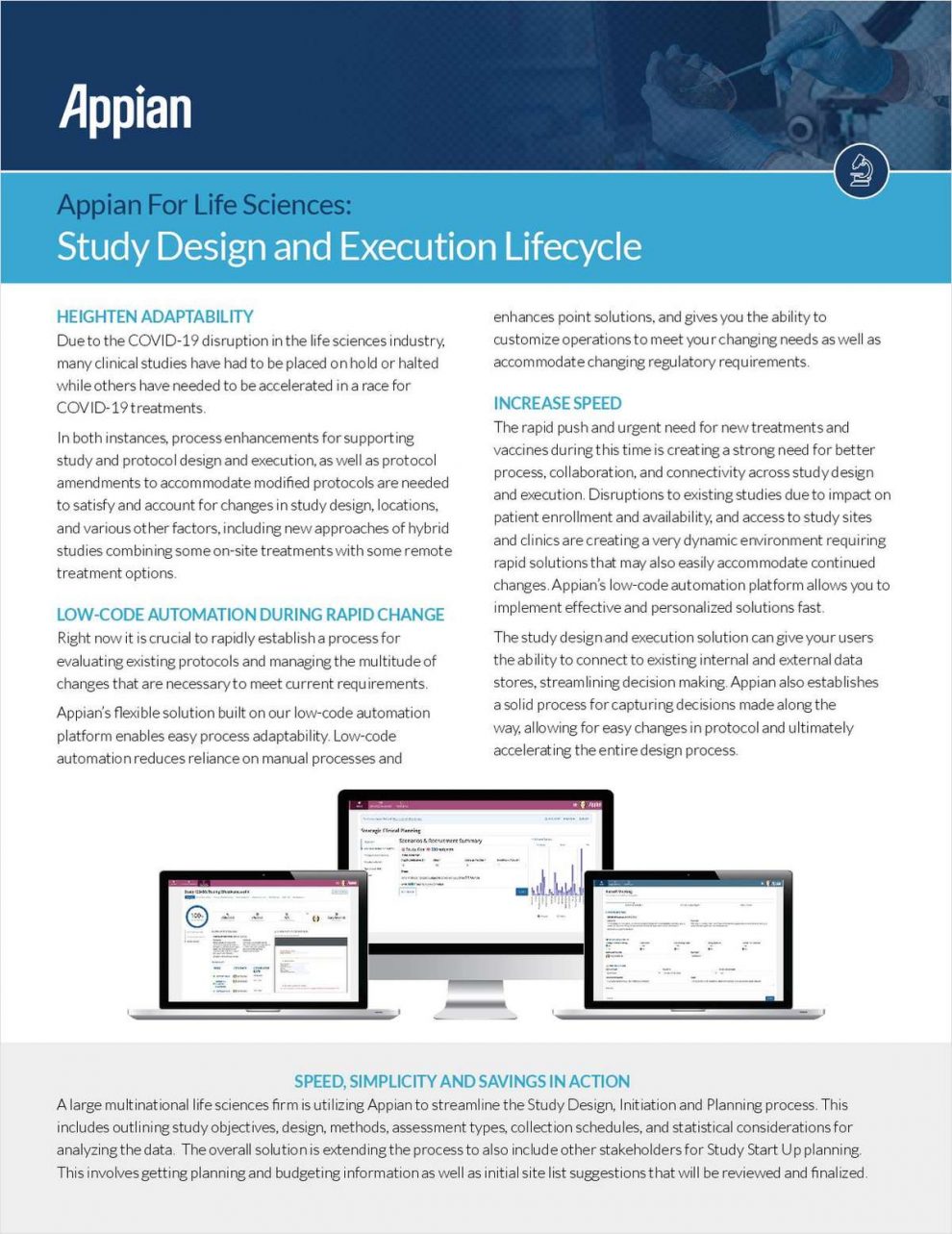
Due to the COVID-19 disruption in the life sciences industry, many clinical studies have had to be placed on hold or halted while others have needed to be accelerated in a race for COVID-19 treatments.
In both instances, process enhancements for supporting study and protocol design and execution, as well as protocol amendments to accommodate modified protocols are needed to satisfy and account for changes in study design, locations, and various other factors, including new approaches of hybrid studies combining some on-site treatments with some remote treatment options.
Download this brief to learn more.












Add Comment